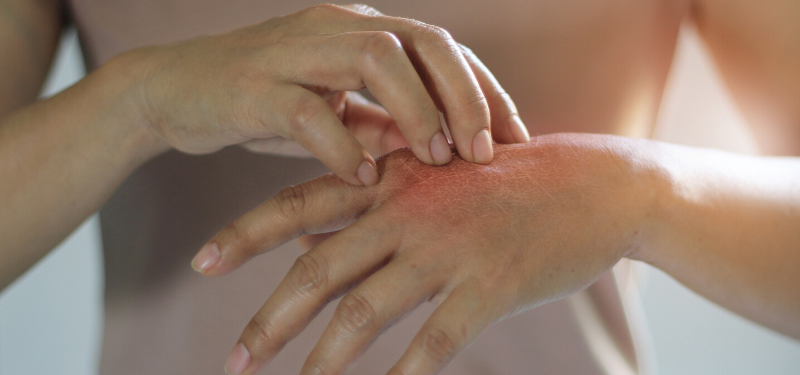
Researchers at Sinai Health have received $1.75 million to study a rare skin disease that affects one in 200,000 newborns worldwide.
The project, lead by a group of scientists from Sinai Health’s Lunenfeld-Tanenbaum Research Institute (LTRI), looks at a specific group of enzymes called tissue kallikreins, thought to play a role in Netherton Syndrome, a rare genetic skin condition.
It is being funded by Toronto Innovation Acceleration Partners (TIAP), along with collaborators Evotec SE and AmorChem II Fund L.P.
Lead project scientist Dr. Ioannis Prassas said their research has implications both in Netherton Syndrome and more broadly in atopic inflammatory dermatitis.
“The identification of skin kallikreins as key driver molecules in the pathology of various skin inflammatory diseases has created exciting new opportunities for novel therapeutics,” Prassas said.
Prassas, along with fellow LTRI scientists Dr. Eleftherios Diamandis and Antoninus Soosaipillai, have spent decades researching the healthy function and disease-related roles of skin kallikreins.
The team’s research shows that the excessive activity of kallikreins expressed in the outer layers of skin is central to the development of skin inflammatory diseases. About two to five per cent of people and up to 30 per cent of newborns suffer from skin inflammatory diseases worldwide.
Netherton Syndrome, a genetic inflammatory disorder, can result in sensitive, dry, and red skin and significantly impede quality of life. Current treatment options are largely ineffective and limited to general immune suppressants and topical lotions that only relieve symptoms.
Dr. Diamandis, a world expert on kallikrein research, said the combined efforts of academic research and the expertise of the LAB150 program, a drug discovery acceleration platform founded by TIAP and Evotec, has advanced the project to the proof-of-concept stage.
“It is exciting to witness the translation of our accumulated knowledge on kallikreins into potential new therapies,” said Diamandis. “We are excited to be working with TIAP, Evotec and Amorchem to move this technology forward.”
The funding will allow the team to complete crucial pre-clinical studies before advancing candidate molecules to clinical testing. They hope to begin generating a kallikrein-targeting candidate for Investigational New Drug-enabling studies by 2021.
