Research Involving Multiple Sites
For Sinai Health studies involving human participants, please refer to the Application Process and Meeting Dates page for details on how to submit to the REB.
CTO Stream offers a single, electronic interface for ethics review, with all studies using the same standardized REB application forms, regardless of which Board of Record is reviewing the study. CTO forms and templates may be found here.
CTO Submission Process
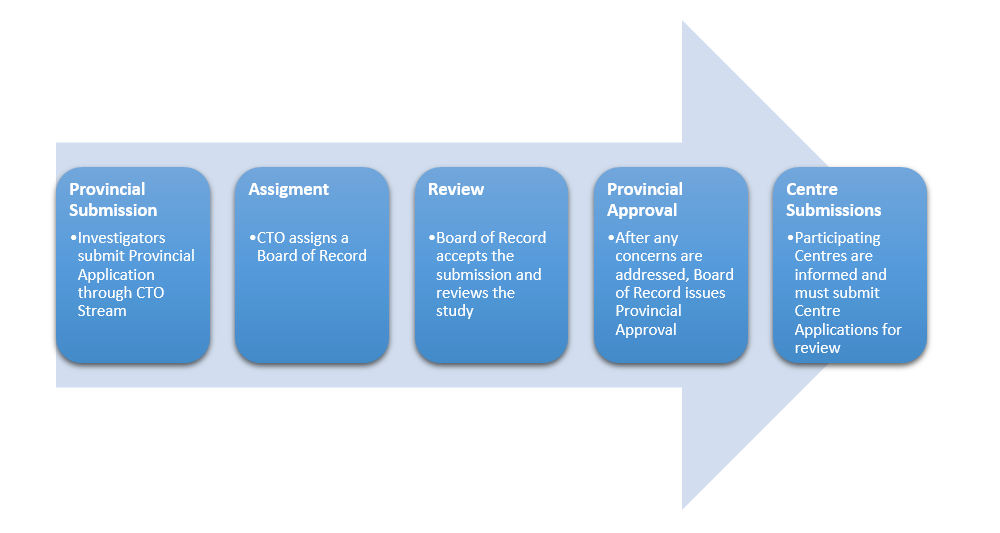
Provincial Review Process for New Study (Lead Site)
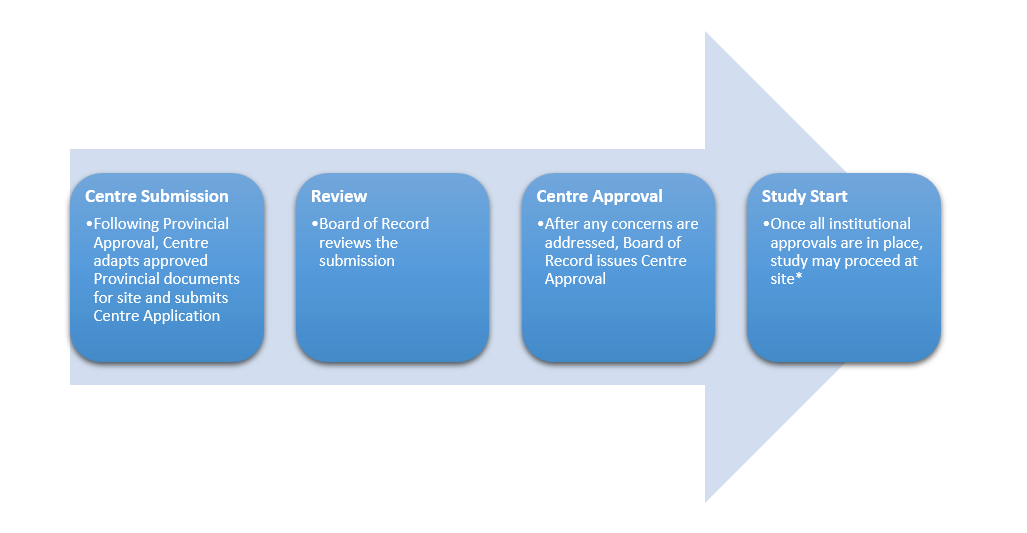
Centre Review Process for Conducting Study at Individual Sites
It is important to note that each site’s institution will have additional review and/or approval requirements (e.g., contracts, departmental impacts, privacy assessments, etc.). These requirements will vary depending on the type of study, and on the structure of the organization where the study is being conducted. All institutional requirements must be met prior to initiating the study at that site.
When completing the Sinai Health Provincial / Centre Initial Application through CTO, you will need to include the name of Sinai Health’s Institutional Representative. This individual is Darlene Homonko, Director of the Office of Technology Transfer and Industrial Liaison [email protected], 416-586-4800 ext. 6380.