Sinai Health’s $10M funding fuels creation of lab-grown organoids for disease research and drug discovery
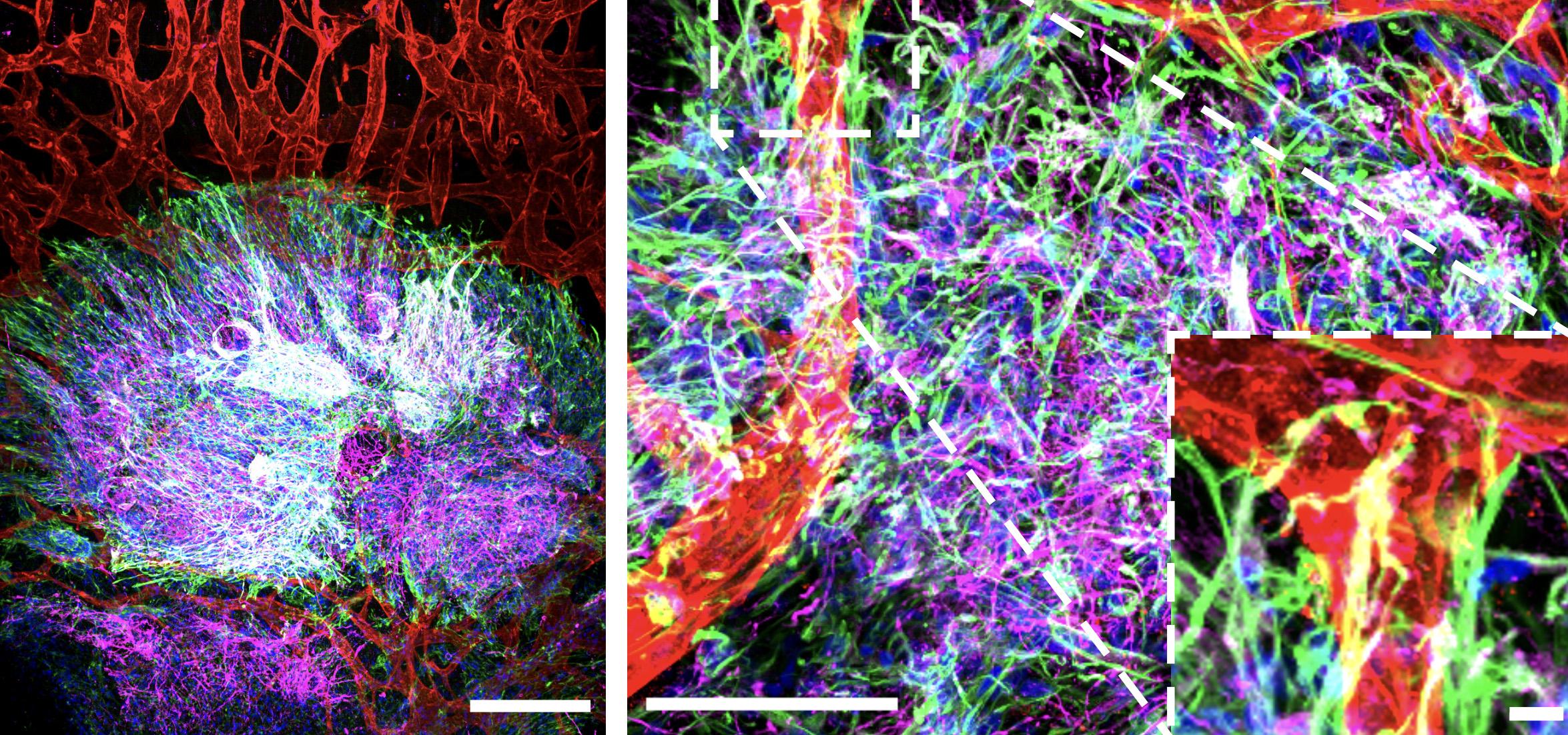
In Drs. Jeff Wrana and Laurence Pelletier’s laboratories at Sinai Health, tiny brain-like tissues are sprouting, growing ever more complex thanks to the blood vessels supporting them.
A feat of tissue engineering, these minuscule structures, called organoids, are derived entirely from human stem cells, and may hold the clues to how the brain develops – and how it unravels in disease.
“Organoids enable us to study normal brain physiology and disorders that range from autism to bipolar disorder to Alzheimer’s in a way that hasn’t been possible before,” said Dr. Wrana, a Senior Investigator at the Lunenfeld-Tanenbaum Research Institute (LTRI), part of Sinai Health.
Scientists around the world are racing to culture three-dimensional organoids for all kinds of tissues, because they mimic human development and disease in a dish and could revolutionize research and drug discovery. Now, thanks to a $10 million investment from the federal and provincial governments to Dr. Wrana, with Dr. Pelletier as co-lead, and their colleagues, Sinai Health will establish a state-of-the-art tissue engineering platform for the development of various organoids, including the brain, gut, lung, and kidney.
The funding will facilitate the acquisition of state-of-the-art scientific equipment, including a single cell biology suite, high-definition microscopes, and advanced 3D bioprinters, to form the Tissue Engineering Design Suite. This endeavour complements LTRI’s existing technology platforms that include a Canadian first – the Nikon Centre of Excellence, which is directed by Dr. Pelletier and focuses on sophisticated microscopy techniques essential for tissue engineering research.
Supported by the Canadian Foundation for Innovation and the Ontario Research Fund, the Tissue Engineering Design Suite will be housed at the Network Biology Collaborative Centre (NBCC), a core research facility hub at the LTRI, where it can be accessed by a wider group of researchers from Sinai Health, the University of Toronto, and beyond.
Human in a dish
Organoids have key advantages as research models over traditional animal testing, particularly in the case of brain research. This is due to their unique ability to reproduce specific aspects of human brain development and architecture. Critically, the organoids allow researchers to listen in on the crosstalk between different cells in their environment, which is important for tissue development and maintenance. When this communication is disturbed, it disrupts tissue architecture and can lead to disease.
“By having these organoid models, we can actually track down in detail how this communication between cells is occurring in a normal state, and then how some kind of perturbation leads to its disruption in disease,” said Dr. Wrana. “This is very difficult to do in in animals because you cannot visualize the earliest events that occur when you disturb this communication,” he said. Organoids also offer the potential for personalized medicine by creating patient-specific tissues, enabling more precise understanding and treatment of individual health conditions.
Collaborating with Dr. Liliana Attisano, a Professor of Biochemistry and Director of the Applied Organoid Core at the Donnelly Centre for Cellular and Biomolecular Research, at the University of Toronto, Drs. Wrana and Pelletier have created a micro-engineered ‘organ-on-a-chip’, which mimics the physiological environment necessary for organoid development, including the integration of blood vessels to enhance tissue maturation. Their next step will be to include immune cells that research has shown are also instrumental for proper tissue development.
“If we can make organoids more like actual tissues by introducing vasculature, immune components, and blood flow, then they should become better and more predictive models of normal physiology and disease,” said Dr. Wrana.
While it remains to be seen if organoids will be better avatars than animal models for research translation, the researchers are excited about the possibilities ahead. Beyond exploring neurological conditions, the tissue engineering platform will enable the cultivation of diverse organoids, which will serve as research models for investigating a range of diseases including colorectal cancer and its metastatic processes, lung fibrosis, polycystic kidney disease, and more.
“This is an exciting time for tissue engineering and regenerative medicine research,” said Dr. Anne-Claude Gingras, co-Director with Drs. Wrana and Pelletier of the NBCC, Director of the LTRI and Vice President of Research at Sinai Health. “The Tissue Engineering Design Suite will ensure that our researchers and collaborators have access to the latest equipment and expertise to be able to make discoveries that could transform our understanding of disease.”